Brief history of autophagy research at NCMM
The Autophagy Team grew out of a research project initiated by Ian G. Mills, a former Group Leader at NCMM, and led by his first postdoctoral fellow, Nikolai Engedal.

Dr. Mills, whose primary research interest is prostate cancer, was studying a kinase, calmodulin-dependent kinase kinase 2 (CAMKK2) which was found to be a regulator of cancer metabolism. This formed part of his research into individual genes and pathways that appeared to have interesting implications as biomarkers or therapeutic targets for prostate cancer.
Literature and past research implied that CAMKK2 had a role to play in autophagy, and in in vivo models of obesity. The appointment of Mills’ first postdoctoral researcher, Dr Nikolai Engedal in 2010, who had a background in membrane trafficking – a fundamental process in autophagy – meant that study of CAMKK2 in this context of autophagy was natural.
After studying prostate cancer it soon became apparent that autophagy itself, how it is regulated in cancer, and how it interplays with stresses of various types in its many forms and processes was an area of research that needed more attention. The team was able to consolidate its position, thanks to the award of a Young Talent Grant from the Research Council of Norway (RCN) to Nikolai Engedal in 2014, and the recruitment of Professor Per Seglen, who Engedal had started collaborating with, as a guest researcher.

The Young Talent Grant and the appointment of Per Seglen meant that the autophagy research team could grow and prosper at NCMM. The team, led by Nikolai Engedal, now has two PhD students nearing completion, and several important papers have already been published.
Professor Per Seglen’s contributions to autophagy research were further recognised in 2017, when he was awarded the King Olav V Prize for Cancer Research.
Team Leader Nikolai Engedal shares some insight into the Autophagy Team’s current research at NCMM
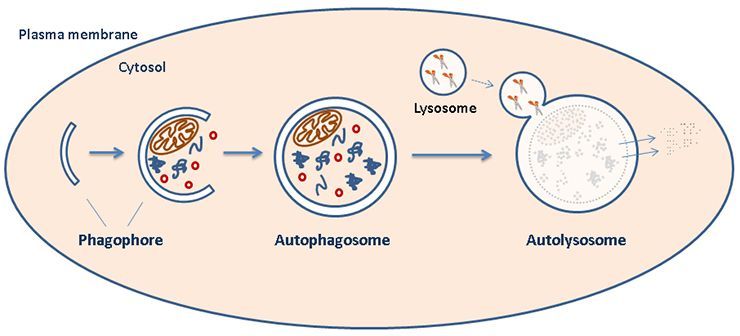
- The Autophagy Team at NCMM is studying processes that lead to intracellular degradation of cytoplasm, collectively referred to as autophagy (Greek for "self-eating").
We aim to understand some of the central regulatory mechanisms and mediators of the pathway, and are particularly interested in the role of autophagy in aspects related to cancer.
What are you working on at the moment?
- One of our major endeavours at the moment is to unravel how changes in the intracellular calcium balance and the so-called "unfolded protein response" (UPR) that is unleashed by endoplasmic reticulum stress is regulating autophagy. Intracellular calcium perturbation and UPR are often interrelated, and both are strongly implicated in cancer as well as other diseases. We have found that calcium pump inhibitors such as the plant compound thapsigargin, totally blocks autophagy (Engedal et al., 2013, Autophagy 9(10):1475-90).
Now we are following up on these findings, and we are trying to understand the relation between autophagic pathways, UPR, and cell death under calcium perturbation conditions. In collaboration with Danish researchers and DANDRITE (a sister centre in the Nordic EMBL Partnership), we are investigating the actions of thapsigargin analogues that constitute the active ingredients of therapeutic cancer prodrugs which are currently at the phase of clinical trials in human cancer patients. In another part of the project, we are studying other UPR-inducers, which do not provoke the same strong calcium perturbation as thapsigargin, and which activate autophagy.
We have already deciphered which of the UPR signalling pathways control autophagy, and we are currently trying to identify specific downstream mediators of UPR-induced autophagy. In two totally different projects, we are studying more basic aspects of the autophagy pathway. First, we are analysing the role of human counterparts of yeast core autophagy machinery genes (identified by last year's Nobel Prize in Physiology or Medicine winner Dr. Ohsumi and others) in starvation, and stress-induced autophagy in human cells. Secondly, we are exploring a novel type of autophagy which has different characteristics and is differently regulated than any type of autophagy that has been described so far.
What do you hope to discover?
- We hope to make fundamental contributions to draw the autophagic pathway map in human cells. Autophagy is much more complex in human cells than in unicellular organisms such as yeast, and several of the human orthologues of the yeast core autophagy machinery components appear to play different, and more diverse roles in human cells. Our team has a unique focus on analysing functional bulk autophagy, and we hope to discover major mediators and regulatory mechanisms related to this process, as well as to understand the commonalities and differences between the bulk process and other more specialized types of autophagy, such as the exclusive autophagic degradation of specific cellular targets.
With respect to calcium and UPR, we hope to understand how the different cellular cues are integrated and translated into autophagic or cell death responses.
Moreover, we hope to pinpoint exactly how and at which steps they feed into the autophagic pathway. This will provide knowledge that is important for future therapeutic strategies and development of drugs to treat cancer and other diseases.
Where do you think your field of research will be in ten years, and what does the future hold for autophagy research at NCMM?
- Autophagy is one of the most rapidly growing fields in cell biology. Autophagic processes appear to play a central role in normal human physiology, as well as in aging and an ever-growing list of diseases. So far, only very few and rather nonspecific clinical drugs exist that target autophagy. Therefore, enormous efforts are currently put in, both by academia and pharmaceutical companies, to produce novel and more specific autophagy-targeting drugs that can be used to treat human diseases.
This endeavour has to go hand-in-hand with basic research on autophagic mechanisms and pathways, because that is the only way that specific and effective drugs can be developed. I think that in ten years from now, we will see even more intense activity in this exciting, and still young research field. Moreover, we will hopefully by then have seen second-generation autophagy-targeting drugs making their first contributions to improve human health.
As a translational centre of molecular medicine NCMM has potential to be an important actor in accelerating this development.